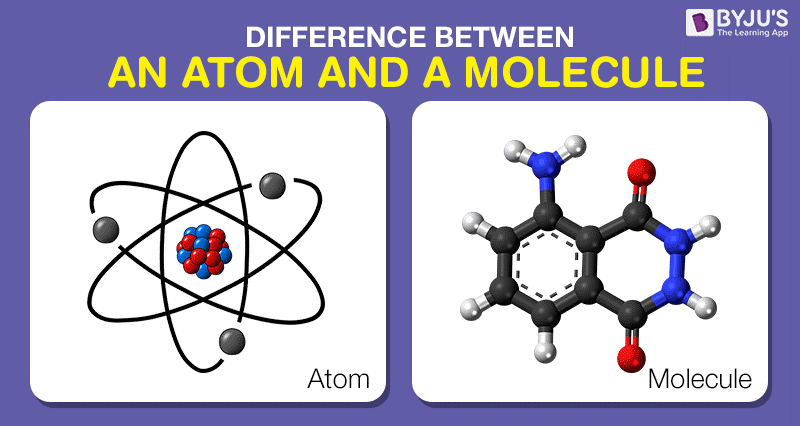
A single molecule is the smallest piece of matter you can have without changing its properties. It is not possible to breakdown the atom further retaining the properties of the element.
Difference Between Atom And Molecule In Tabular Form
Start your trial now.
. Molecules are always more than one atom and the atoms are chemically bonded together. A molecule is a bunch of atoms combined. How does an atom differ from a molecule.
WaterH2O containing hydrogen and oxygen. For example Hydrogen is. Carbon or a compound- this consists of different types of atoms chemically bonded together eg.
In what ways are they similar. Molecules can be elements - a set of the same atom eg. An atom is the smallest part of an element that you can get without splitting the nucleus of the atom.
An atom differ from a molecule by. An atom is the smallest component of an. 11 rows So while an atom is its own separate entity a molecule is what you get when those atoms bond.
Conversely a molecule comprises of two or more identical or different atoms combined chemically. For a long time scientists believed that atoms cannot be broken into smaller pieces. Mixtures contain more than two substances in different proportions.
An atom is the smallest unit of matter that cant be chemically broken down and molecules are formed from two or more bonded atoms. Describe how an atom differs from a molecule. The atoms can be same or different elements.
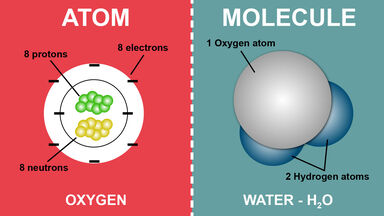
Atoms comprise of the nucleus which contains protons and neutrons and electrons. It is characteristic and unique for each element. Because an atom is the smallest unit of matter that cannot be broken down by chemical means and molecule is a group of atoms held together by covalent bonds.
Weve got the study and writing resources you need for your assignments. The atomic mass also referred to as the atomic weight is the number of protons and neutrons in an atom. Differ from atom give exampleFAQhow does molecule differ from atom give exampleadminSend emailDecember 20 2021 minutes read You are watching.
Atoms of an element that have differing numbers of neutrons but a constant atomic number are termed isotopesIsotopes shown in Figure 1 and Figure 2 can be. Describe how the molecule whose formula is NO is different from the molecule whose formula is N2O. Atoms are the building blocks of molecules.
The atomic number is the number of protons an atom has. And a compound is a type of molecule in which the types of atoms forming the molecule are different from each other. Difference Between Atom and Molecule An atom is smallest particle in an element that has the properties of the element.
The significant difference between atom and molecule is that an atom is regarded as the tiniest particle that constitutes matter. Molecules are neutral particles made of two or more atoms bonded together. For example the atoms of element gold cannot be broken down.
Those different substances in a mixture are mixed together but they are not joined with each other. Step 1 of 4. In what ways are they similar.
1 - How does an atom differ from a molecule. 1 - Classify each of the following as an element a. And atoms are the base structure of molecules.
The shape of an atom is spherical whereas the molecules can be linear angular or rectangular in shape. Molecules are made of more two or more atoms joined together. 7 rows What is the difference between Atom and Molecule.
1 - How are the molecules in oxygen gas the. An ion is a positively or negatively charged particle. An atom is the smallest part of an element while a molecule is two or more atoms held together by covalent bonds.
First week only 499. Each substance in a mixture keeps its own properties. On the contrary a molecule is the combination of two or even more smallest units ie atoms that are chemically bonded together.
Whenever the names atoms and molecules are taken there occurs confusion that how the two are different and what. 1 - A sulfur atom and a sulfur molecule are not. A molecule of water is made View the full answer.
When two or more atoms link up they create a molecule. Not all molecules are compounds because some molecules such as hydrogen gas or ozone consist only of one element of only one type of. This is in contrast to N 2 O which consists of two nitrogen atoms and one oxygen atom.
Everything around us is made up of either molecules or atoms. Atoms are not visible to the naked eye and are the basic building blocks. Oxygen is an example of a diatomic molecule found in Earths atmosphere.
A molecule is formed when two or more atoms of an element chemically join together. The main difference between atom and molecule is their size. Most objects in the universe are made of atoms.
1 - Classify each of the following as an element a. Distinguish between covalent bonds and ionic bonds. A molecule has a definite molecular weight and a unique chemical formula.
Molecules are every where. Atoms are single neutral particles. An atom is the smallest component of an element.
However they are able to dig deep and find particles smaller. In this article we will discuss the difference between atom and molecule with regard to their chemical as well as physical properties. 2 rows The difference between atoms and molecules is that atoms are the smallest unit of matter and.
1 - Many of the items you purchase are mixtures of. Give an example of a diatomic molecule found in Earths atmosphere. All substances that are present in the nature are made up of minute entities called atoms.
Solution for How does an atom differ from a molecule.

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube

Atoms And Molecules Difference Between Atom And Molecule Youtube
0 Comments